Antibody Development and Engineering
Antibody-based proteomic analysis requires the generation of novel, high-affinity probes. Proteins identified through mass spectrometry analysis can be validated using antibody reagents, but more importantly, these antibodies can also be used to assess target expression in a broader range of tissues. Core activities include:
Generation of caveolae- and tissue-specific monoclonal antibodies
Phage display libraries
High-throughput antibody generation platform
Nanostreptabodies
Nanoparticle Development and Production
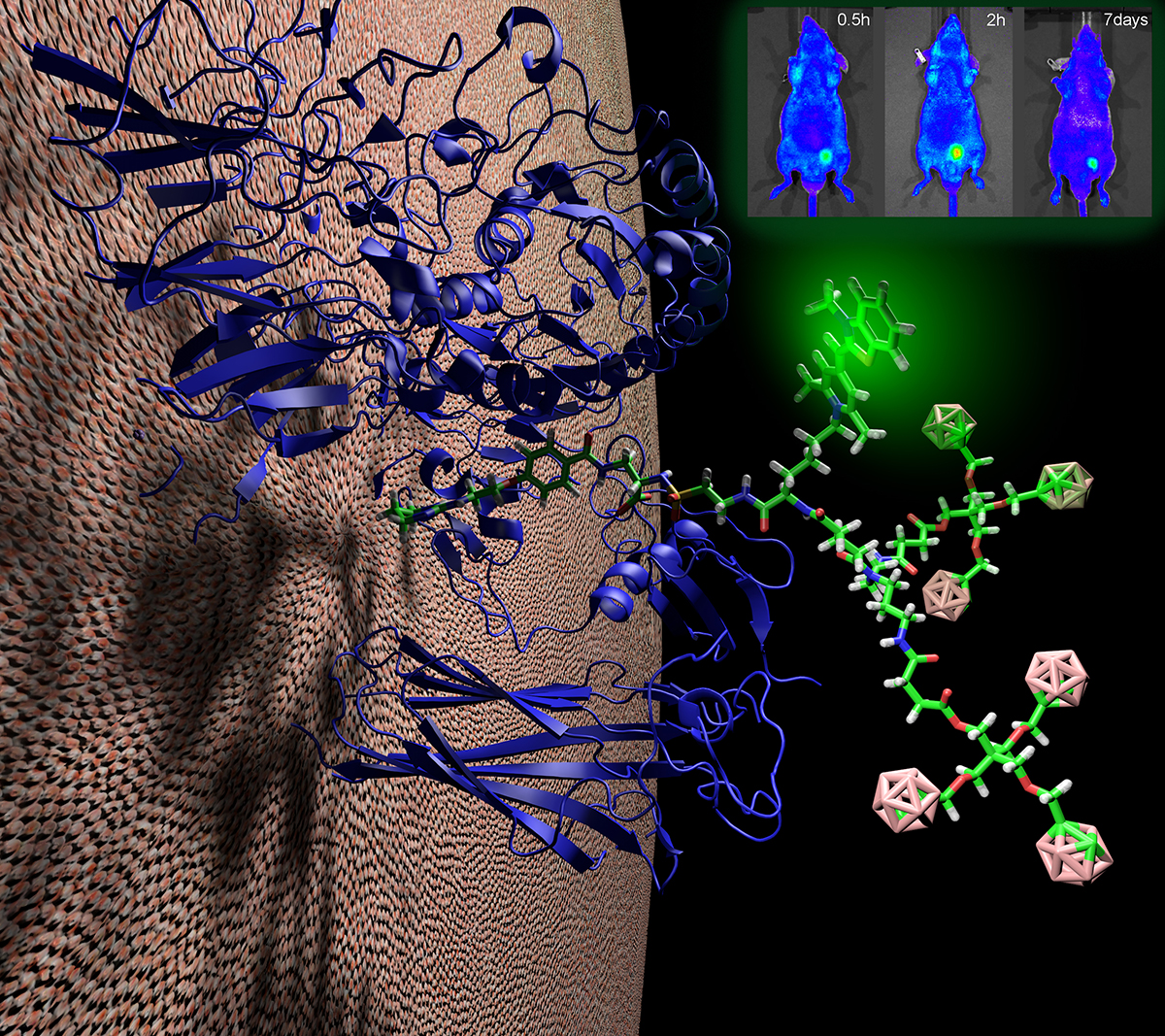
Generating novel antibody drug conjugates, radio-immunotherapies, nanoparticles, and imaging agents that specifically target and penetrate deep into target tissues is key to rendering them more efficacious and less toxic through enhanced caveolae-mediate delivery. The Nanoparticle Core focuses on the development, optimization and analysis of a range of multifunctional nanoparticles with different physical properties and links them to targeting antibodies for various clinical applications. Nanoparticles currently under development include:
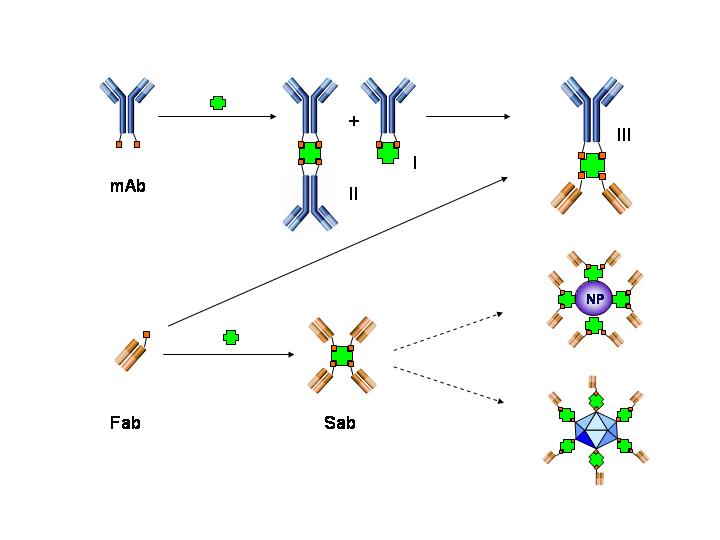
Immunocomplexes
Dendrimers
Polymer-based nanoparticles
Imaging
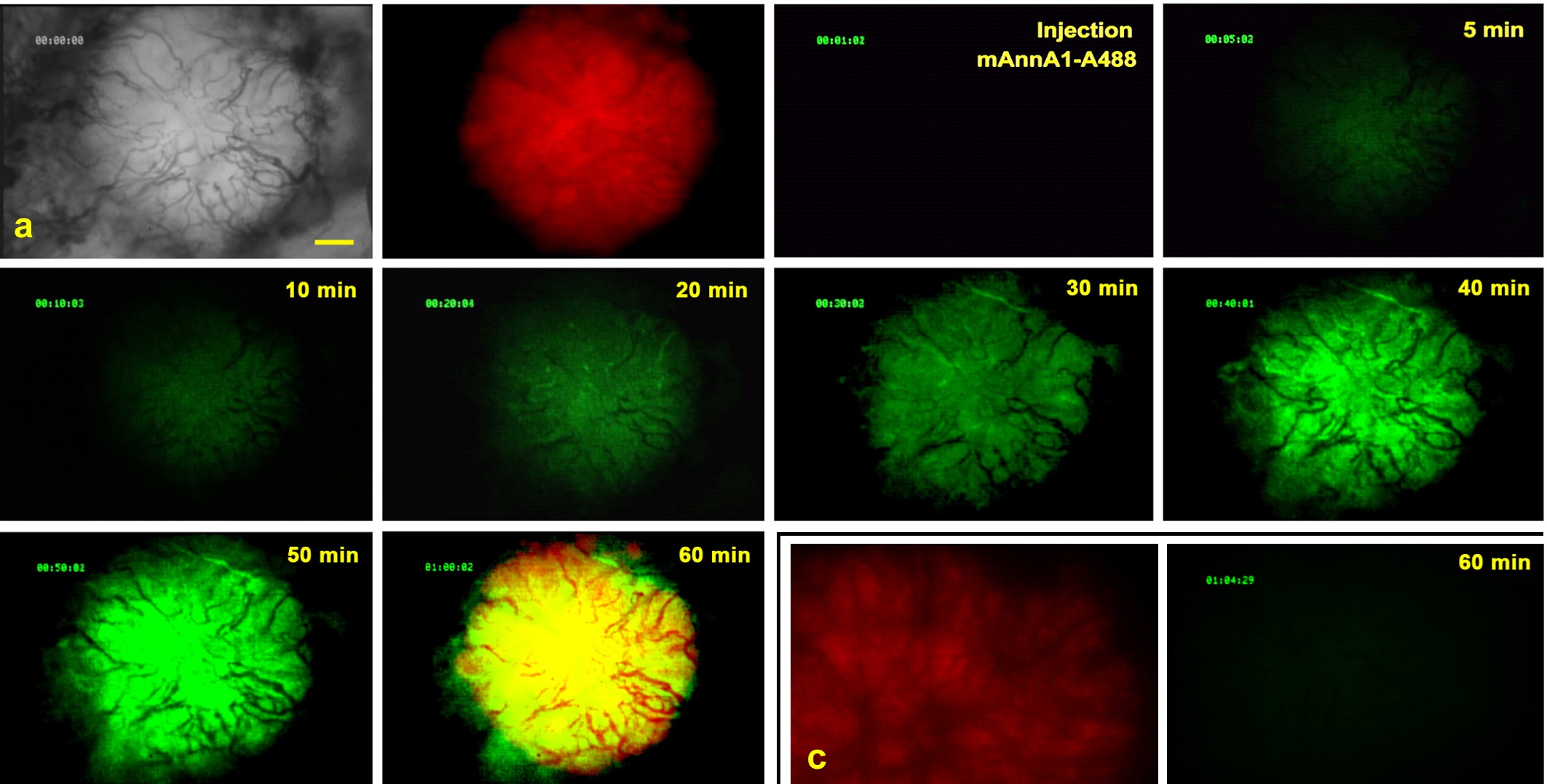
The main goal of the Imaging Core is to determine the extent of in vivo targeting and assess the therapeutic impact of intravenously injected immunocomplexes conjugated with various drugs and active agents. Imaging modalities include:
Intravital Microscopy
Gamma Scintigraphy and SPECT-CT
Static Imaging
Mass Spectrometry
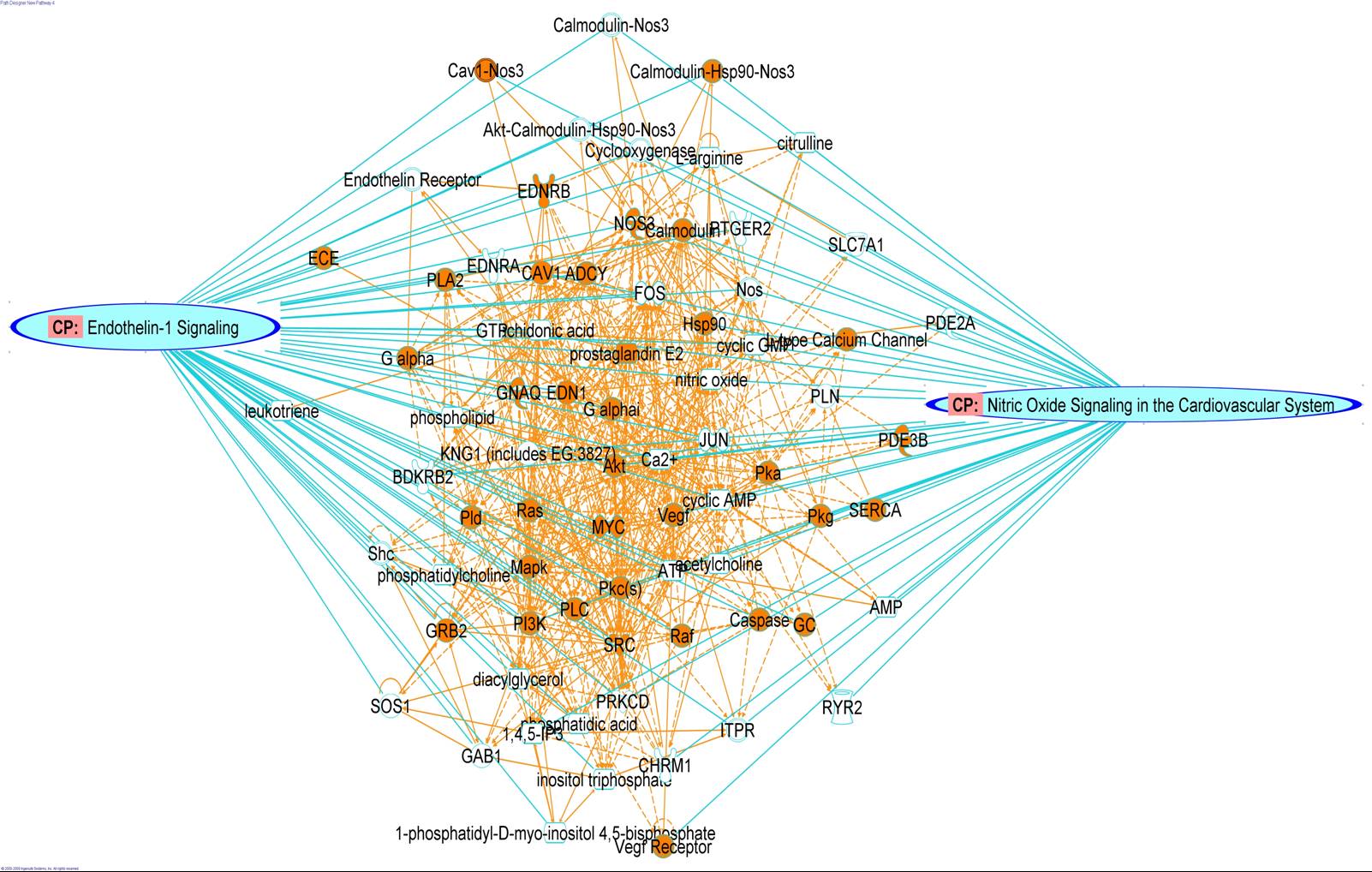
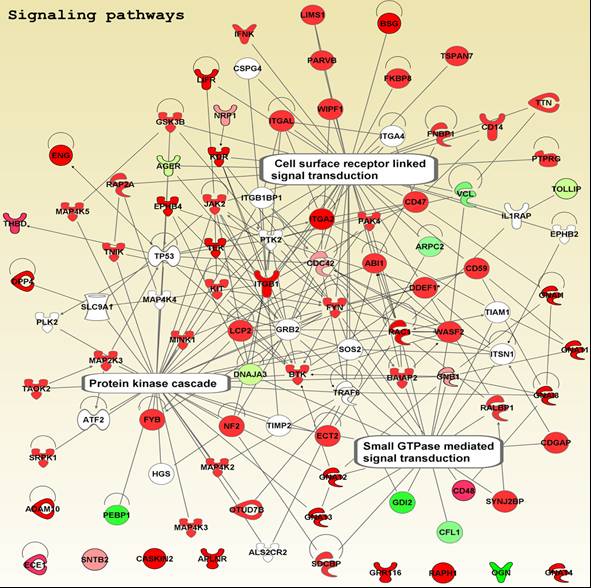
Scientists at PRISM have extensive experience in mass spectrometry-based approaches to protein identification. They have developed a comprehensive classification of proteins at the luminal surface of endothelial cells and have pioneered novel methods to normalize data that allows reliable quantification and direct comparison. Key activities include:
Protein expression analysis
Analyzing post-translational modifications
Bioinformatics
Systems Analysis
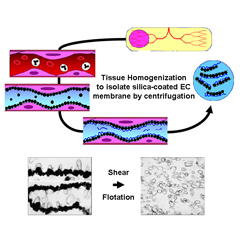
Tissue Subcellular Fractionation
Isolating high-quality proteins, membrane microdomains, organelles and cells directly from tissues is critical for downstream applications. Because endothelial cells are only a minor component of tissues, global proteomic analysis of whole tissues is unlikely to yield much useful information, especially if the aim is to identify disease-induced biomarkers. Core capabilities include:
Isolation of specific proteins, proteins complexes, membrane microdomains, cell organelles, and cells directly from healthy and diseased tissues.
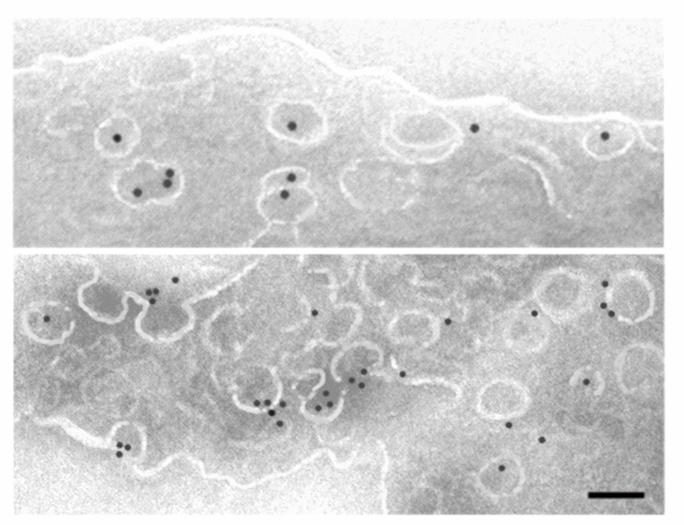
Specialized techniques for isolating the luminal surface of endothelial cells in vivo using silica-based nanoparticles and subfractionation.